Key takeaways:
- The appeals court’s order partially granted a request to freeze parts of a Texas judge’s order, preserving access to the medication abortion drug mifepristone.
- The ruling narrows the decision by US District Judge Matthew Kacsmaryk, keeping the drug on the market while an expedited appeal plays out.
- The court’s ruling will keep the drug available while the case is being appealed, but under more restrictive conditions than the FDA had authorized.
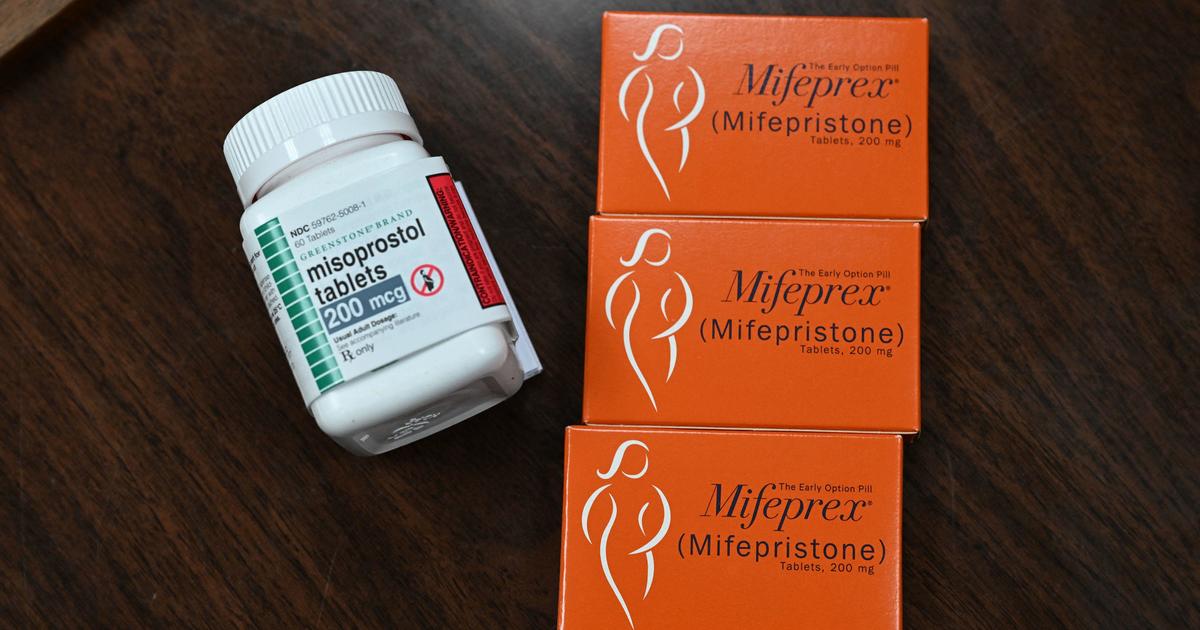
A federal appeals court has partially granted a request to freeze parts of a Texas judge’s order that would have suspended the US Food and Drug Administration’s approval of a medication abortion drug. The court’s ruling preserves access to the drug, mifepristone, but under tighter rules that would allow the drug only to be dispensed up to seven weeks, not 10, and not by mail.
The 2-1 vote by a panel of three judges on Wednesday night narrows the decision by US District Judge Matthew Kacsmaryk last Friday that had completely blocked the FDA’s approval of the drug following a lawsuit by mifepristone’s opponents. The appeals court’s order will keep the approval in effect and the drug on the market while an expedited appeal plays out.
The FDA approved the drug, mifepristone, more than two decades ago. It is commonly used in combination with another drug, misoprostol, to terminate a pregnancy up to 10 weeks. The drug is typically dispensed in person at a doctor’s office, but the FDA had recently authorized it to be dispensed by mail.
The Justice Department and the drug’s manufacturer had requested that the appeals court put Kacsmaryk’s ruling on hold. However, the US 5th Circuit Court of Appeals is leaving in place parts of the ruling that halted changes the FDA made to the drug’s label, including the authorization to dispense it by mail.
The appeals court’s ruling will keep the drug available while the case is being appealed, but under more restrictive conditions than the FDA had authorized. The court’s decision is expected to be appealed to the Supreme Court.


Be First to Comment