Key takeaways:
- The Food and Drug Administration (FDA) is considering the approval of a new drug, called exa-cel, which could be the first cure available for many patients with sickle cell disease.
- Blood and bone marrow transplants are currently the only option for curing sickle cell disease, and are limited to a minority of patients who can get donors that are close genetic matches to them.
- If approved by the FDA, exa-cel could provide a universally available, potentially curative option for individuals with sickle cell.
The Food and Drug Administration (FDA) is considering the approval of a new drug, called exa-cel, which could be the first cure available for many patients with sickle cell disease. The drug, developed by Vertex Pharmaceuticals, uses a gene editing technology called CRISPR and could provide an alternative to the current treatment options, which are limited to a minority of patients.
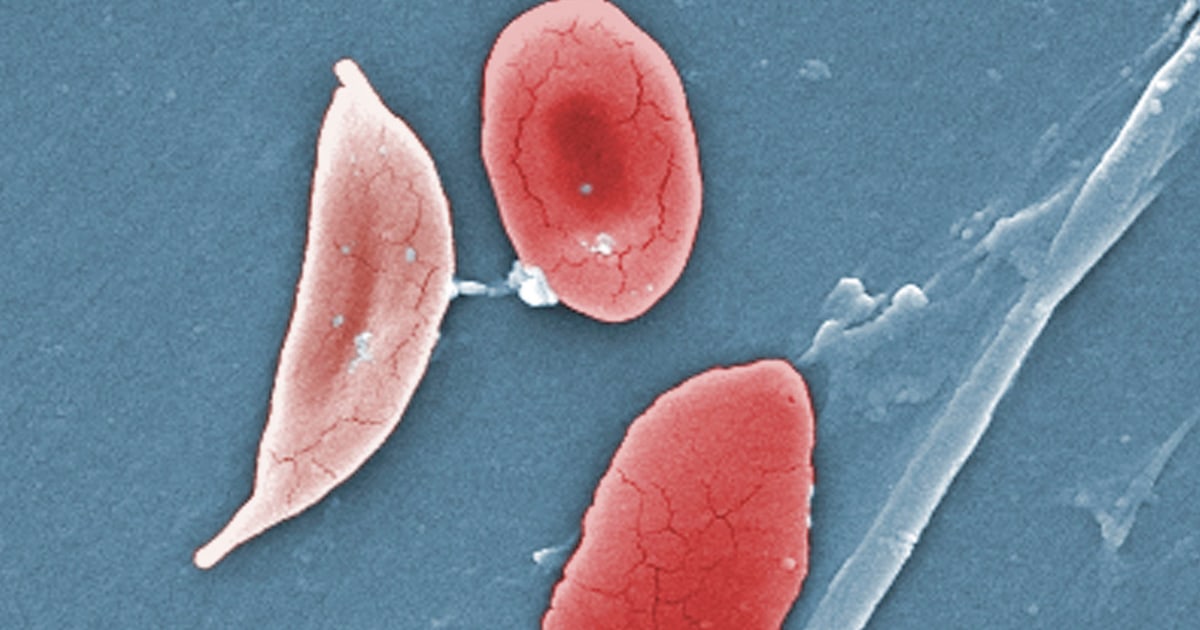
Sickle cell disease is an inherited blood disorder that affects an estimated 100,000 people in the U.S., most of whom are Black, according to the Centers for Disease Control and Prevention. The illness causes the body’s red blood cells, usually disk-shaped, to take on a crescent or sickle shape. This can lead to anemia, pain, and organ damage.
A panel of the FDA’s outside advisers voiced few concerns Tuesday during a daylong meeting wrestling with any potential risks posed by the new therapy. Blood and bone marrow transplants are currently the only option for curing sickle cell disease, the National Institutes of Health says, and are limited to a minority of patients who can get donors that are close genetic matches to them.
If approved by the FDA, exa-cel could provide a universally available, potentially curative option for individuals with sickle cell. The FDA is expected to make a decision on the drug in the coming months. If approved, it would be the first drug to use the groundbreaking gene-editing tool CRISPR.


Be First to Comment